Table of Contents
Are you searching to gather information about polar covalent bond? Electrons are distributed differently in ionic and covalent bonds. Covalent bonds can be nonpolar or polar and respond to electrostatic charges. Ionic bonds, like those in table NaCl, are due to attractive electrostatic forces among their positive Na+ and negatively charged Cl- ions.
We compared atoms to puppies and electrons to bones in our analogy of how bonding goes. In ionic bonding, each puppy begins with an electron ossein, but one puppy acts like a thief and steals the other puppy’s bone. Now one puppy has two electron osseins, and one puppy has none. Because the electron bones in our analogy have a negative charge, the puppy robber becomes negatively charged due to the additional bone.
The puppy that missed its electron bone becomes charged. Because the puppy who lost his bone has the opposing charge of the thief puppy, the puppies are held together by electrostatic forces, a bit like sodium and chloride ions! In covalent bonds, like chlorine gas (Cl2), both atoms share and keep tightly onto all other’s electrons. In our analogy, each puppy again starts out an electron bone. However, rather than 1 puppy stealing the other’s bone, both puppies hold onto both bones.
Any covalently bonded molecules, like chlorine gas Cl2, fairly share their electrons (like two fairly healthy puppies each holding both bones). Other covalently bonded molecules, like fluoride gas (HF), don’t share electrons correspondingly. The fluorine atom operates as a rather stronger puppy that pulls a touch harder on the shared electrons (see Fig. 3-1c).
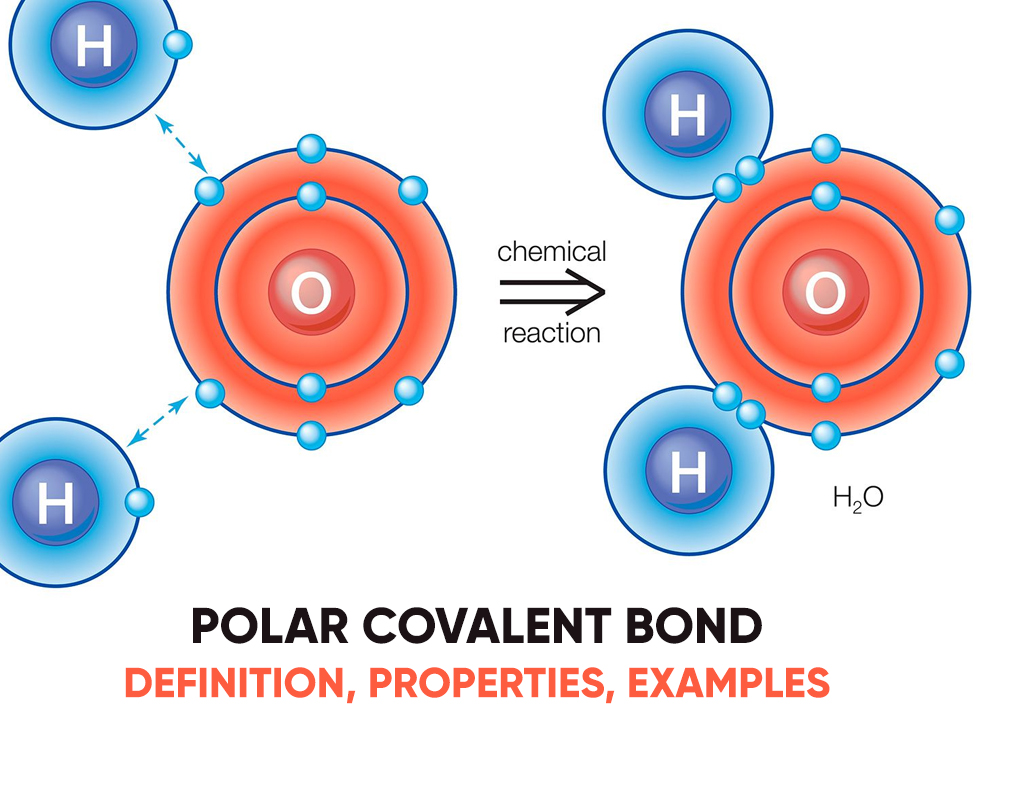
Albeit the electrons in fluoride are shared, the fluorine side of a water molecule pulls tougher on the charged shared electrons and becomes charged. The atom features a slight charge because it cannot hold as tightly to the opposing electron bones. Covalent molecules with this kind of uneven charge distribution are polar. Molecules with polar covalent bonds have a positive and negative view.
Polar Covalent Bond – Definition
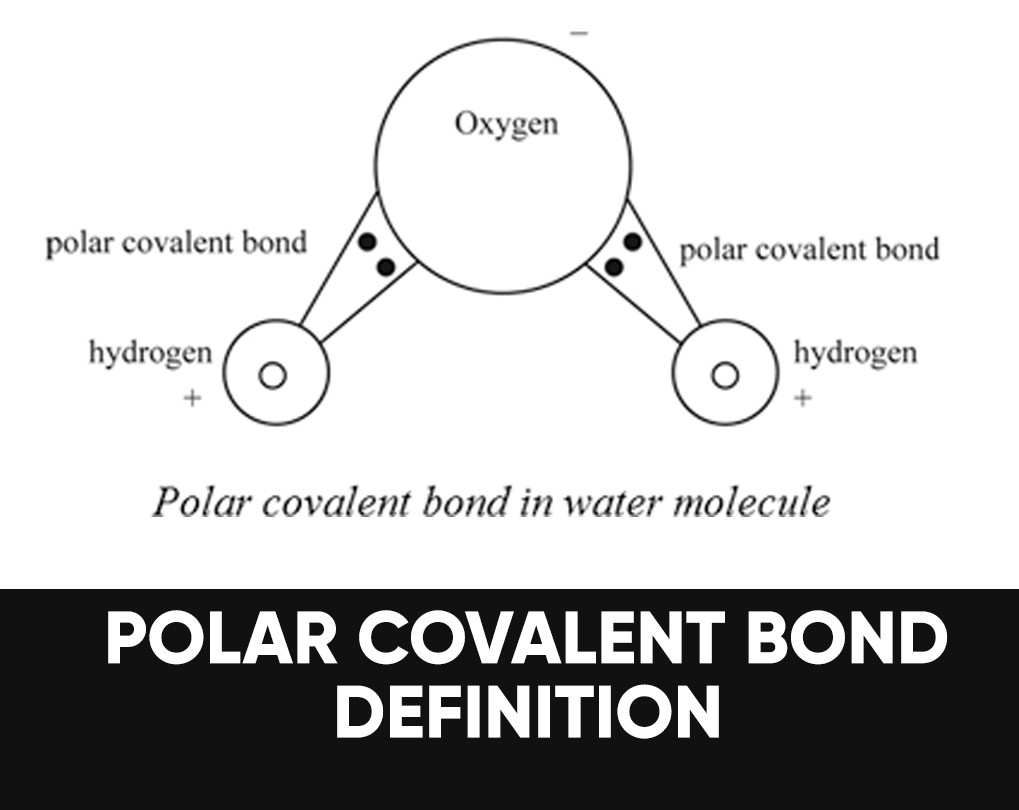
A polar bond may be a chemical bond among two atoms where the electrons build the bond are unfairly shared. This root the molecule to possess a small electrical moment where one end is slightly positive and therefore the other is somewhat negative. The charge of the electrical dipoles is a smaller amount than a full unit charge, in order that they are treated as partial charges and denoted by delta plus and delta minus. Because positive and negative charges are removed within the bond, molecules with polar covalent bonds interact with dipoles in other molecules. This produces dipole-dipole intermolecular forces between the molecules.
Polar bonds are the carved line between pure covalent bonding and pure ionic bonding. Pure covalent bonds (nonpolar covalent bonds) share electron sets equally between atoms. Technically, nonpolar bonding only occurs when the particles are identical to each other (e.g., H2 gas). Still, chemists consider any bond between atoms with a difference in electronegativity less than 0.4 to be a nonpolar covalent bond. Carbon dioxide (CO2) including methane (CH4) are nonpolar molecules.
In ionic bonds, the electrons in the bond are actually donated to one atom by the other (e.g., NaCl). Ionic bonds form between atoms when the electronegativity difference between them is more significant than 1.7. Technically ionic bonds are simply polar bonds so that the terminology can be confusing.
Just recognize a polar bond relates to a type of covalent bond where electrons aren’t equally shared, and electronegativity values are slightly different. Polar covalent bonds form among atoms with an electronegativity difference between 0.4 and 1.7.
Examples of Molecules with Polar Covalent Bond
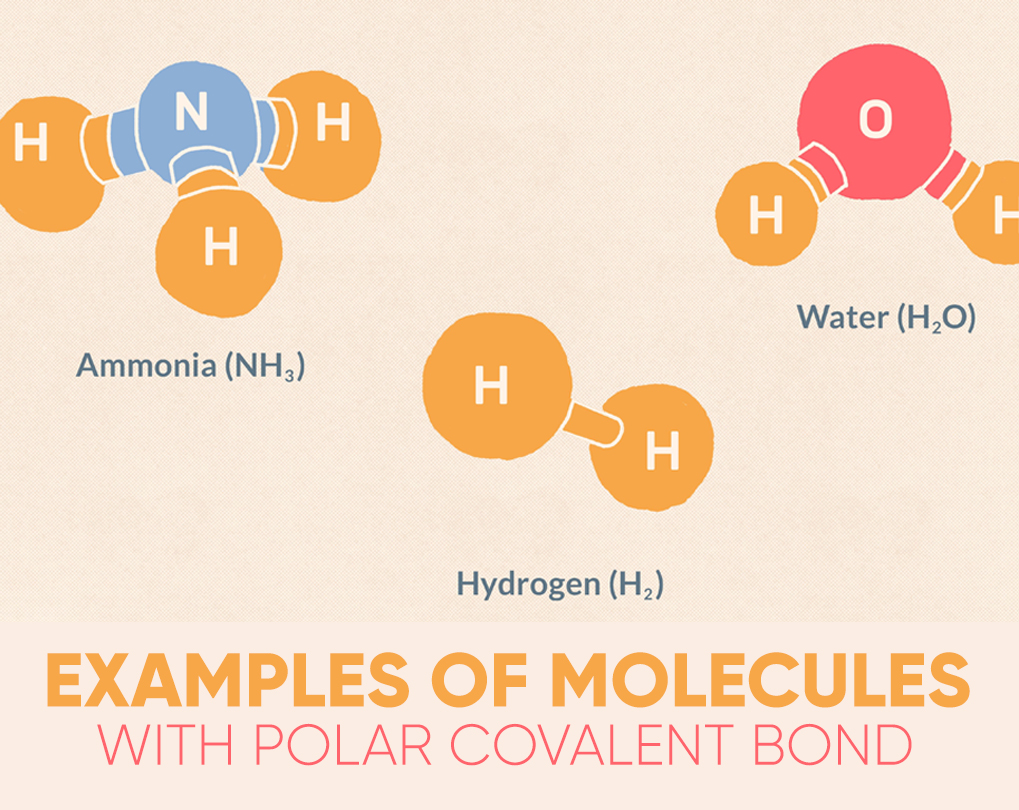
Water (H2O) is a polar bonded molecule. The electronegativity amount of oxygen is 3.44, while the electronegativity of hydrogen is 2.20. The inequality in electron distribution accounts for the best shape of the molecule. The oxygen side of the molecule has a net negative charge, while the two hydrogen atoms (on the different side) have a net positive charge.
Hydrogen fluoride (HF) is another instance of a molecule that features a polar chemical bond. Fluorine is that the more electronegative atom, therefore the electrons within the bond are more intimately related to the fluorine atom than with the atom. A dipole forms with the fluorine view having a net charge and therefore the hydrogen side was having a net charge. Fluoride may be a linear molecule because there are easily two atoms, so no other geometry is probably going.
The ammonia molecule (NH3) has polar covalent bonds among the nitrogen and hydrogen atoms. The dipole is so that the nitrogen atom is more negatively charged, with the three hydrogen atoms all on one side of the nitrogen atom with a positive charge.
Which Elements Form Polar Bonds?
Polar covalent bonds form among two non-metal atoms that have sufficiently different electronegativities from one another. Because the electronegativity values are slightly different, the bonding electron pair isn’t equally shared between the particles. For instance, polar covalent bonds typically from among hydrogen and the other non-metal. The electronegativity value between metals and non-metals is considerable, in order that they form ionic bonds with one another.
FAQs
[wps_faq style=”classic” question=”Q: How do you see if a bond is polar covalent?”]A: To determine the polarity of a covalent bond acting numerical terms, get the difference between the electronegativity of the atoms; if the effect is between 0.4 and 1.7, then, generally, the bond is polar covalent.[/wps_faq][wps_faq style=”classic” question=”Q: How does a polar covalent bond form?”]A: A Polar Covalent Bond is formed when the shared electrons between atoms are not equally shared. This happens when one atom has a higher electronegativity than the atom it is sharing with. As a consequence of polar covalent bonds, the covalent compound that information will have an electrostatic potential.[/wps_faq][wps_faq style=”classic” question=”Q: What is polar and nonpolar?”]A: Partly ionic bonds are called polar covalent bonds. Nonpolar covalent bonds, with equal sharing of the bond electrons, arise when the electronegativity of the two atoms are similar.[/wps_faq][wps_faq style=”classic” question=”Q: What is the difference between polar and nonpolar?”]A: Nonpolar bonds form among two atoms that share their electrons equally. Polar bonds form when two bonded atoms share electrons unfairly.[/wps_faq]